top of page

Product Licensing
Product Licensing
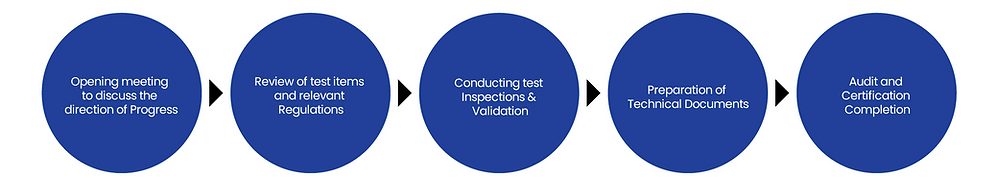
Service Progress Procedure
Medical Device Classification

Grade-by-Grade Procedures [Summary]
-
Grade 1: If you register and apply online at the 'Medical Device Electronic Civil Complaint Window', the 'Korea Medical Device Safety Information Institute' will process the report and issue a report certificate.
-
Grade 2: After the 'Medical Device Technical Document Review' is completed and a Notice of Conformity is issued, you can receive a certificate from the 'Korea Medical Device Safety Information Institute'.
-
Grade 3/4: If clinical trial data is not required, a 'medical device technical documentation review' is conducted. If clinical trial data is required, a 'clinical data review' is conducted. If it is deemed appropriate, a license can be issued by the 'National Institute of Food and Drug Safety Evaluation'.







bottom of page